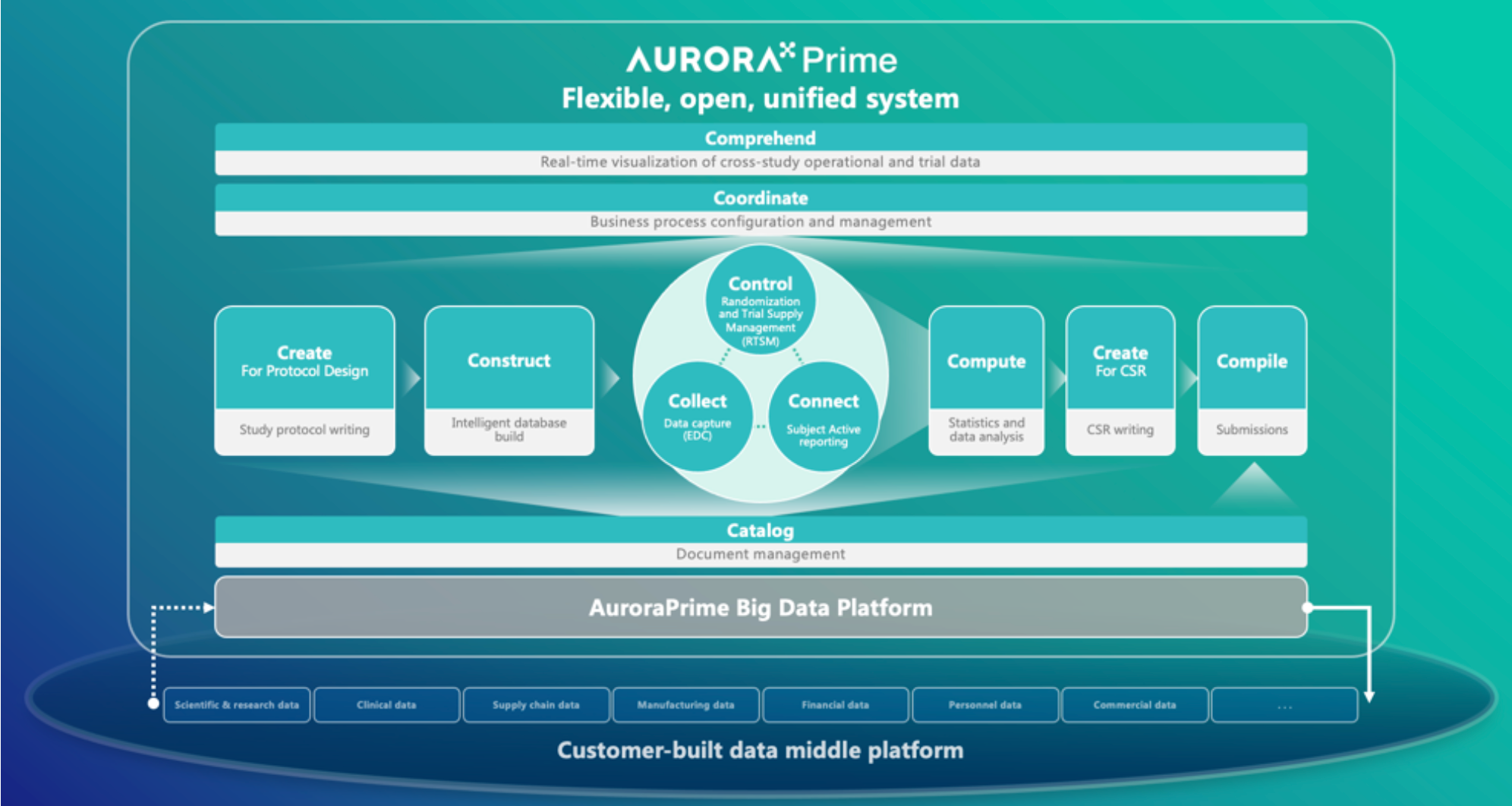
Product Strategy
Medical Monitoring Config Benchmarking Analysis
2022.12 - Now
Researcher & Designer
20 mins
2022.12 - Now
Researcher & Designer
20 mins
As more and more companies and organizations in phanmaceutical industry are adopting R to their statistical programming process, it is possible that more and more clinical studies and programmers will be utilizing R Studio Project.
Why would one choose Medical Monitoring and opt for a configurable experience?
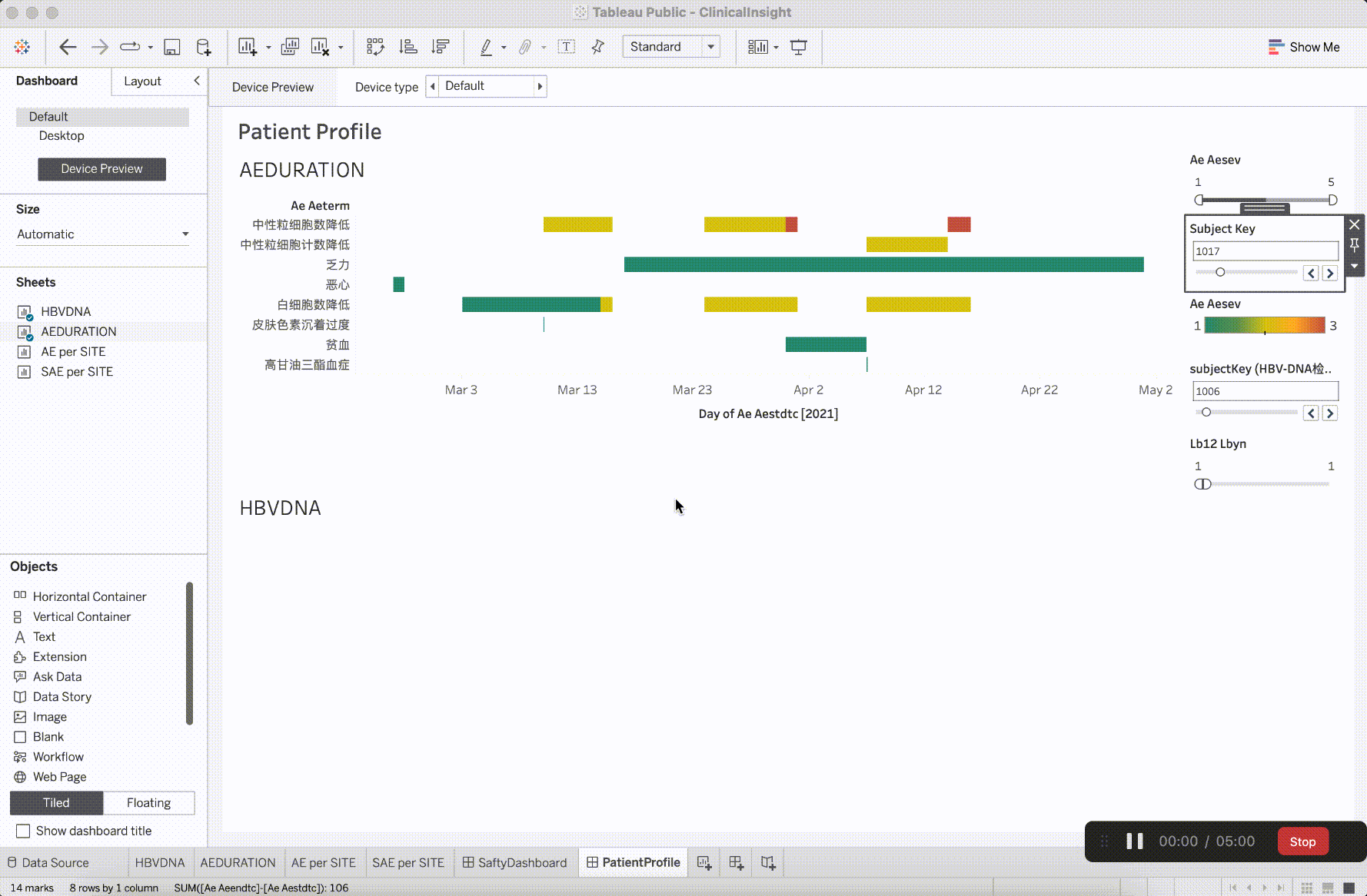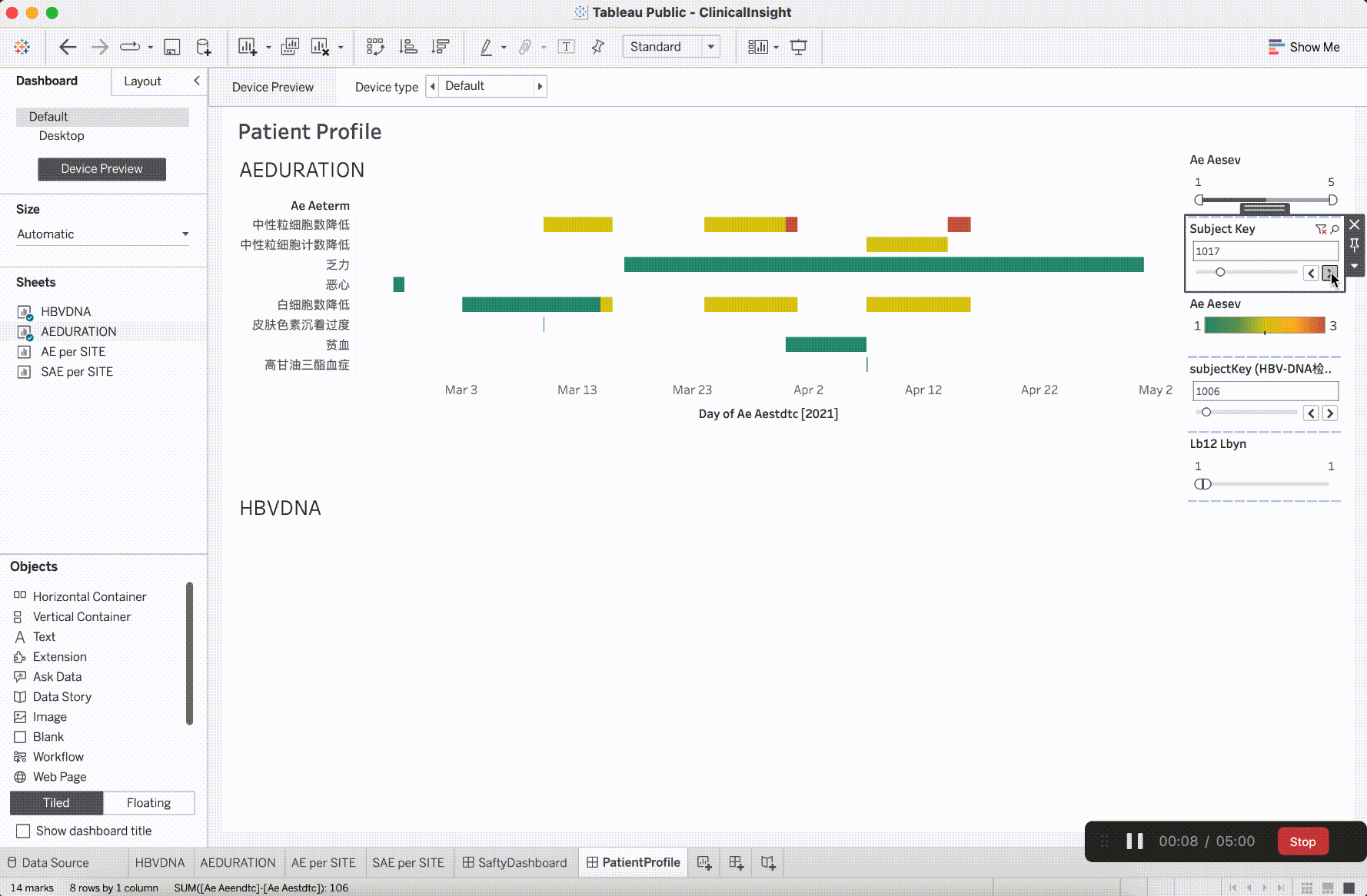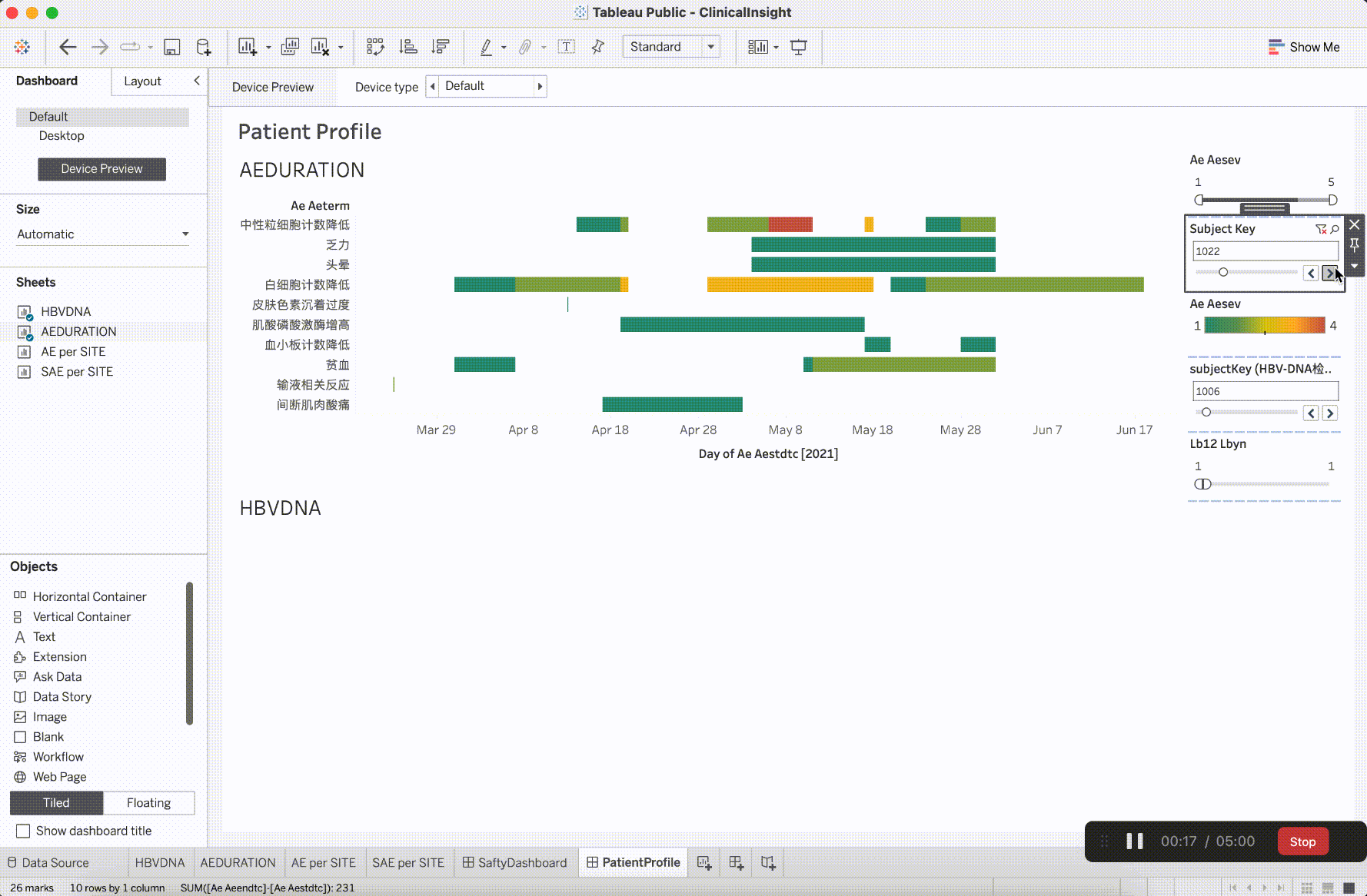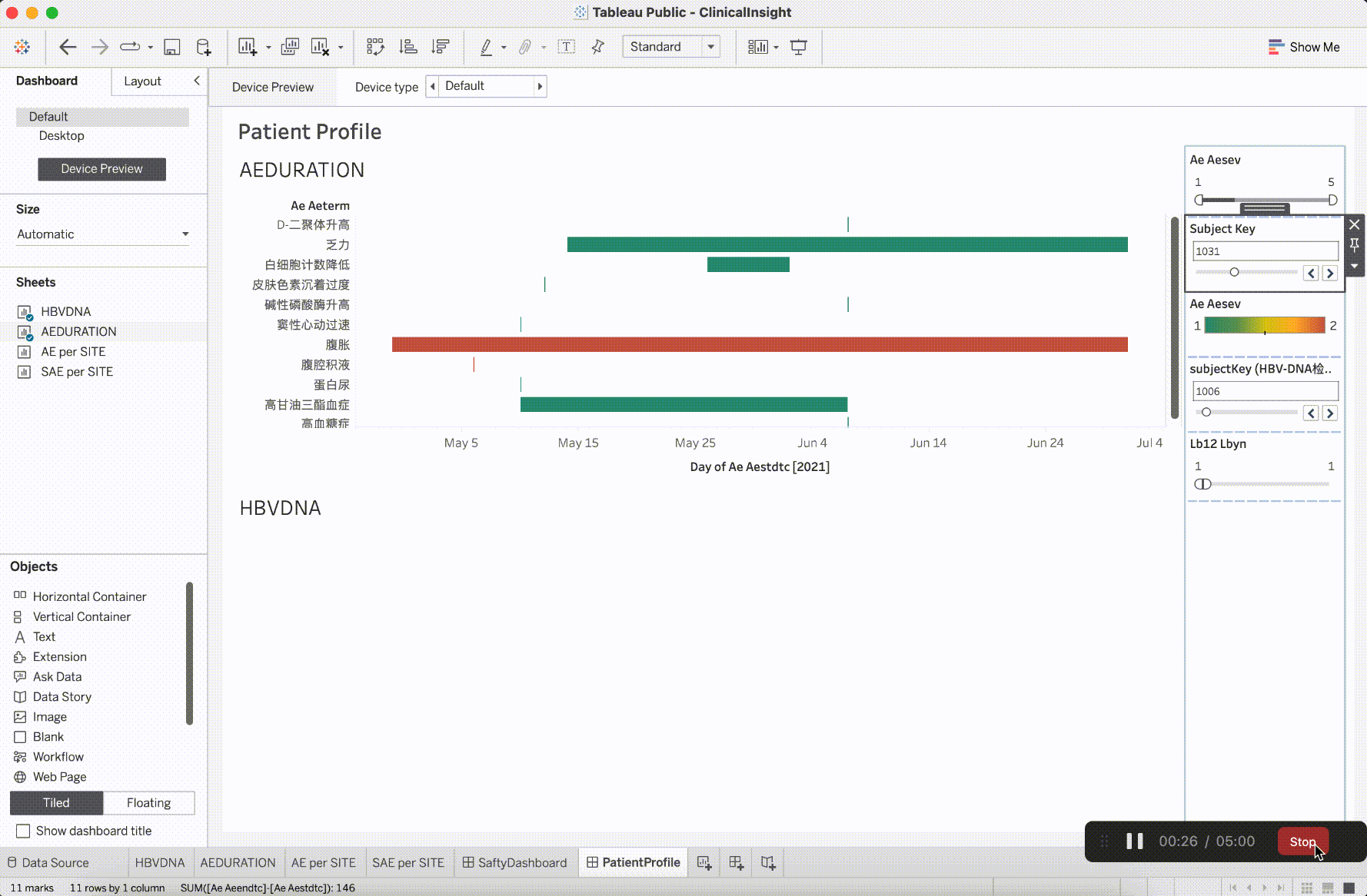
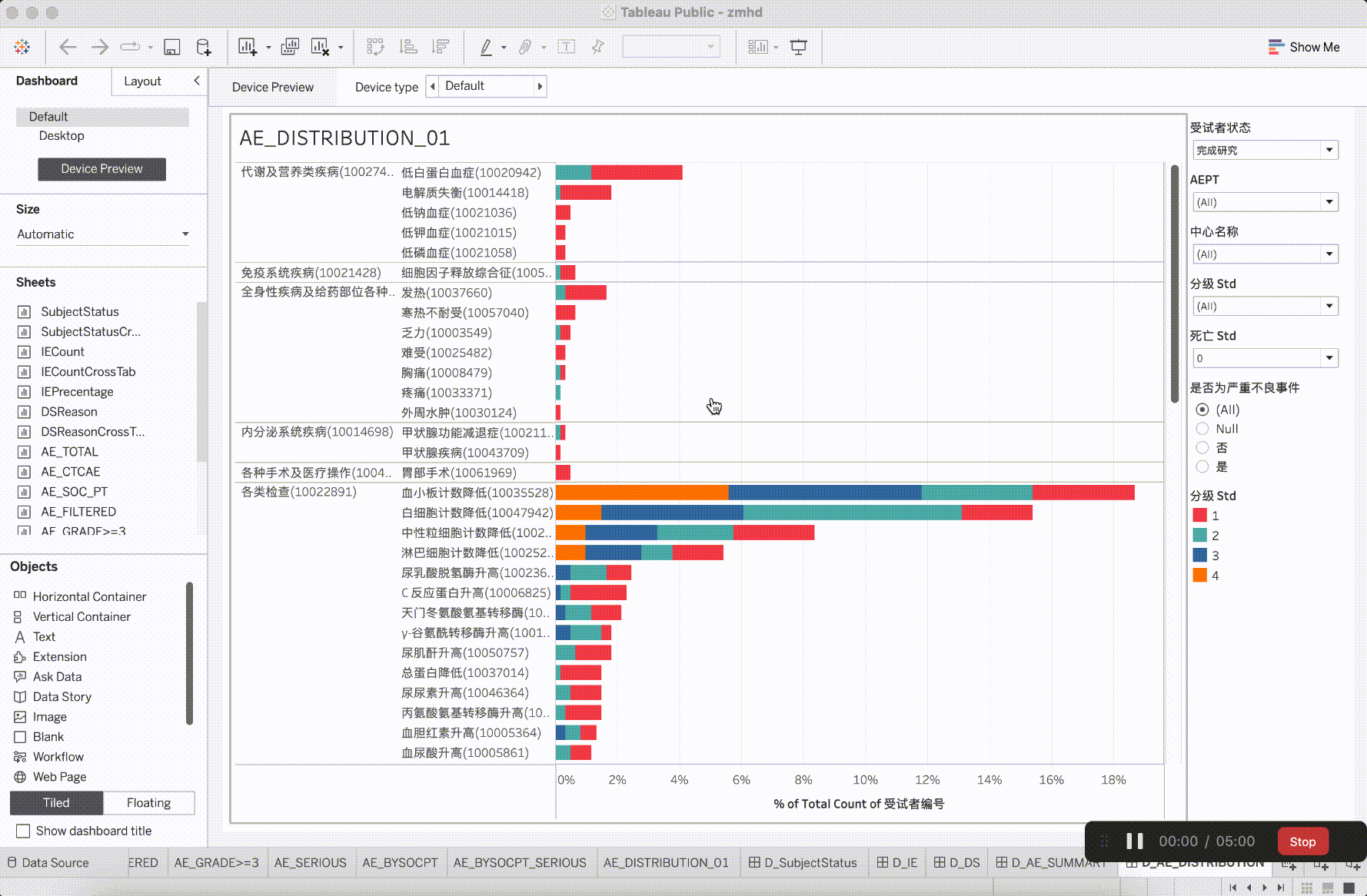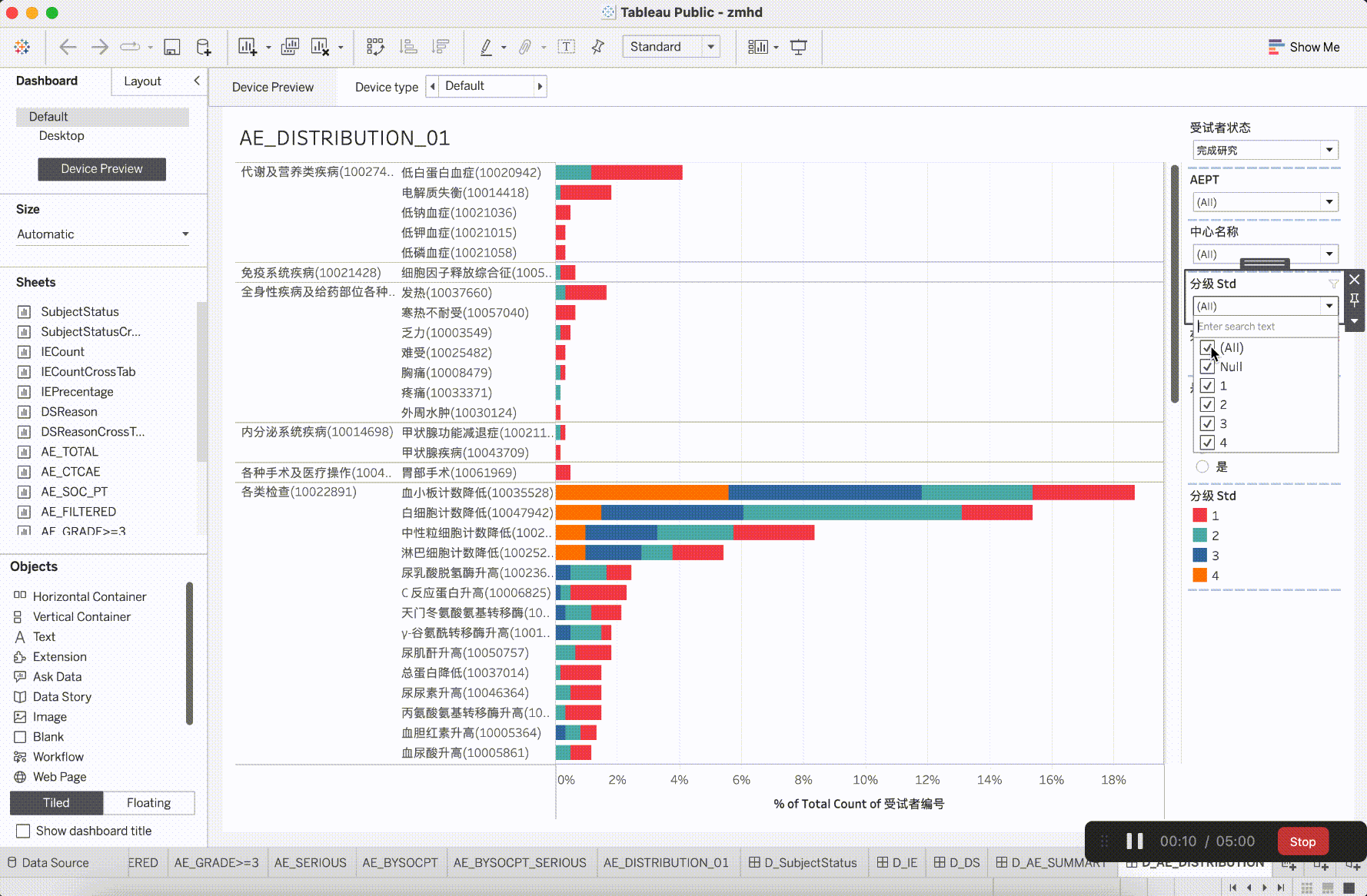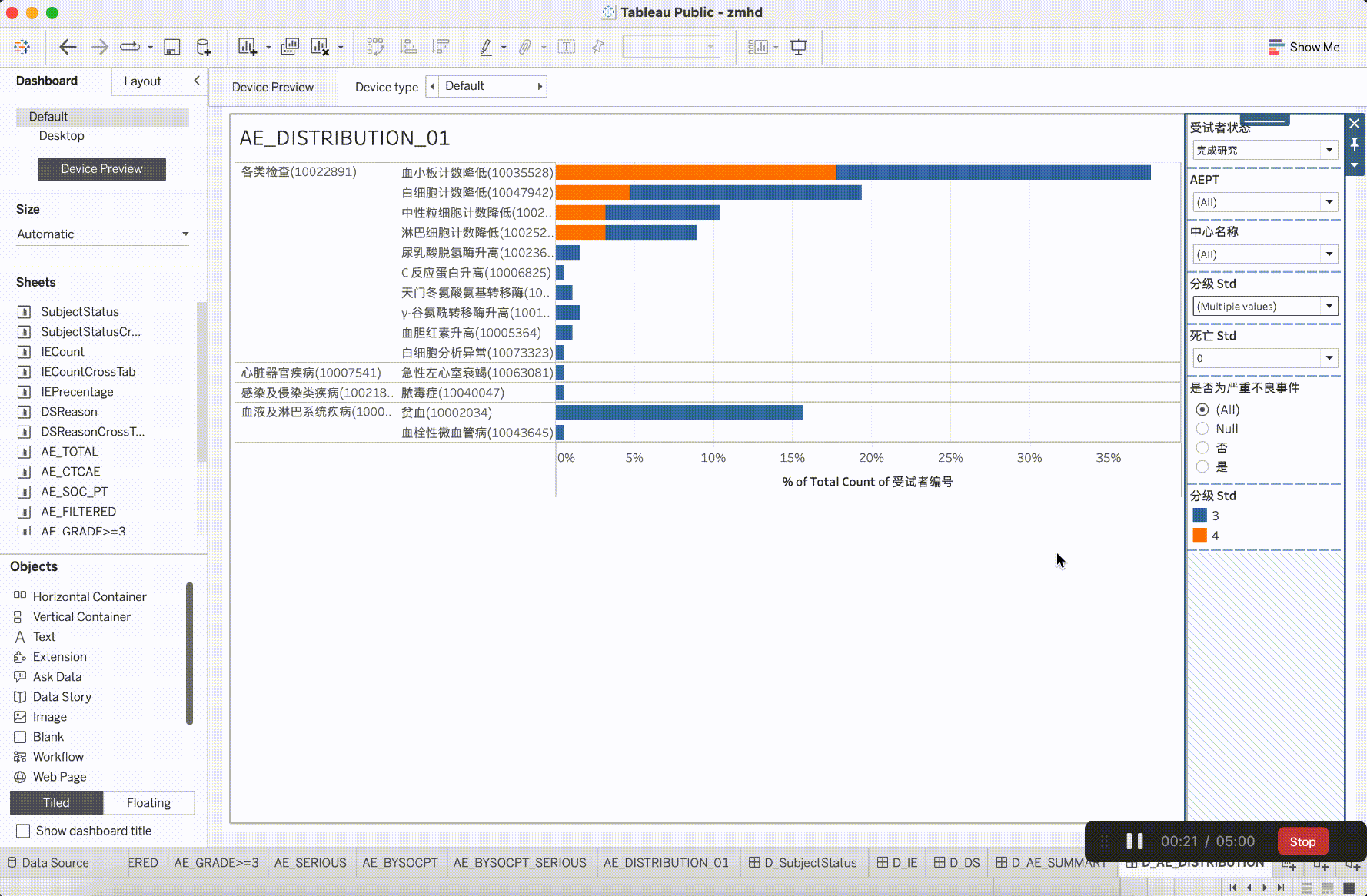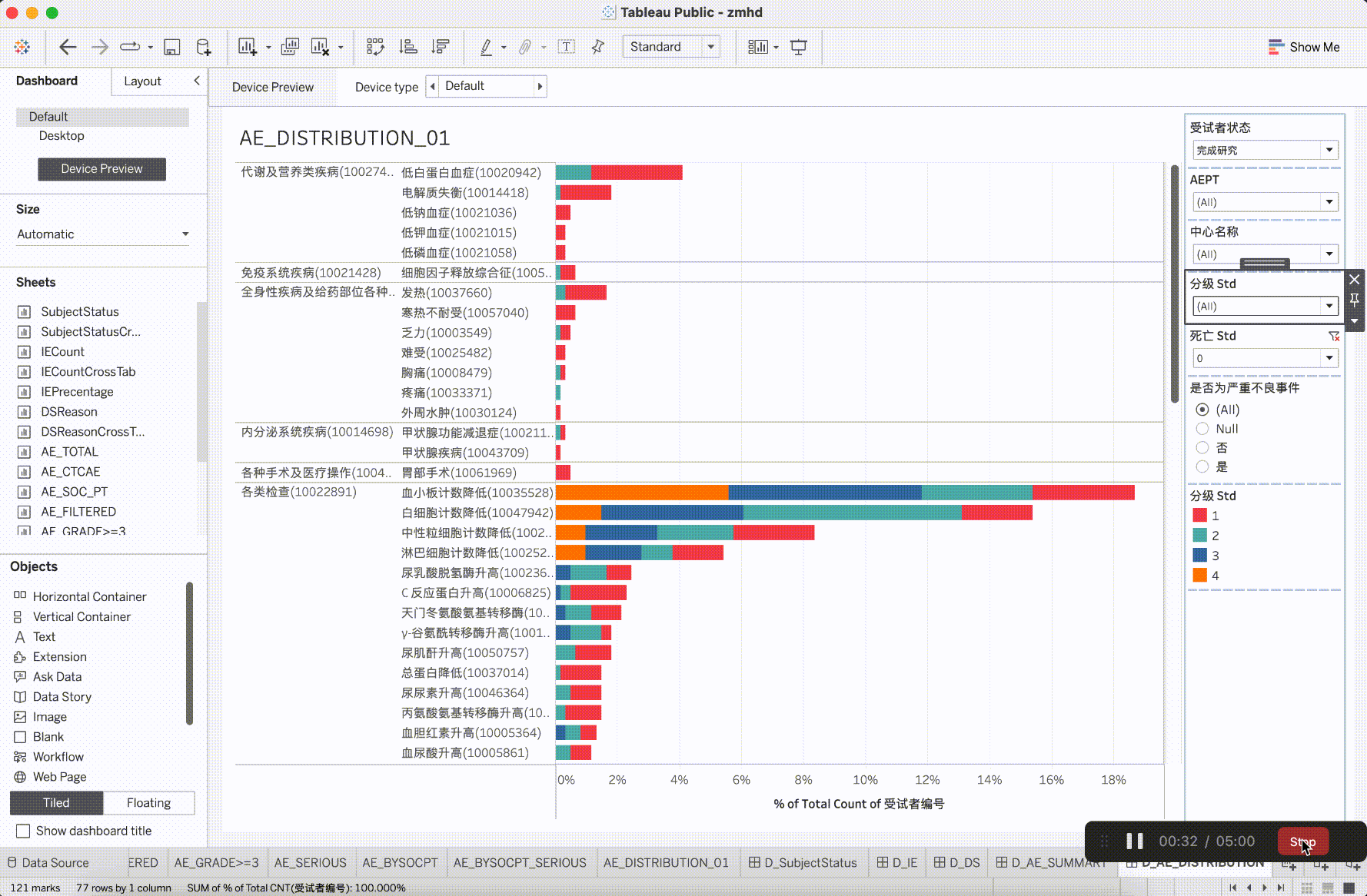
In clinical trial research, the verification content of medical monitoring involves a significant focus on insights from safety data. It is the aspect that requires the most close attention during the trial operation. The most characteristic events to be closely monitored are Adverse Events (AE events).
Adverse Events (AE events) are often monitored and presented based on Preferred Terms (PT) and System Organ Classes (SOC), utilizing various dimensions for categorization. This includes segmentation by site, CTCAE (Common Terminology Criteria for Adverse Events) grade, whether it is an AESER (Adverse Event of Special Interest), whether it is a TEAE (Treatment-Emergent Adverse Event, occurring during treatment), and its association with the investigational drug, among other dimensions. In addition to conventional clinical data visualization plots such as bar, scattered, line, boxplot, and butterfly plot, more advanced data models have emerged to gain insights into the correlations between AEs. For example:
After integrating the R tidyverse, ggplotly, and Plotly into the platform, a solid foundation for presentation in medical monitoring scenarios has been established. The computational capabilities showcased here have initiated exploration and development in the realm of configurability.
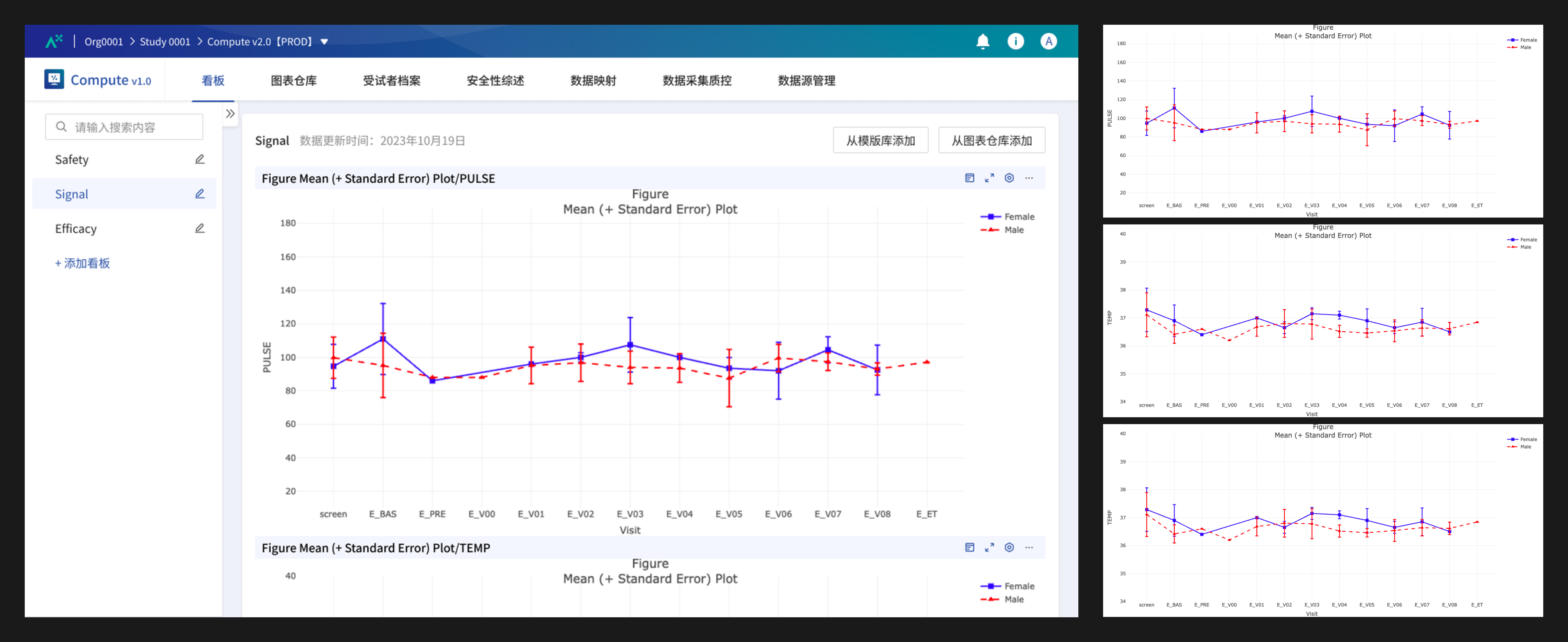
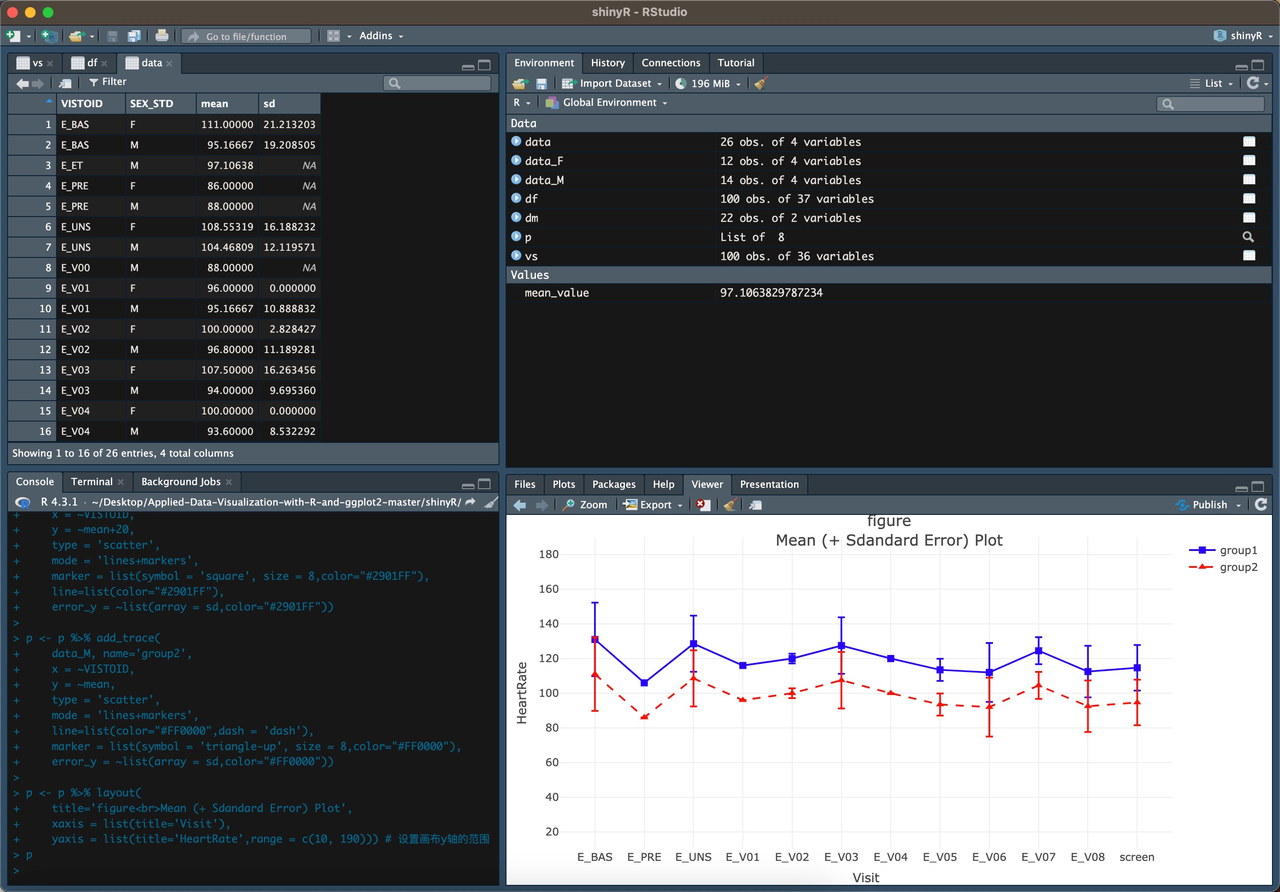
The Vital Sign Error Bar Plot fills missing values using the mean, displays visits in a fixed order, and excludes specific visits, such as unplanned visits.

1. Research and analyze the commonly used functionalities of medical monitoring tools, understand the current and future business needs, and explore possible mechanisms to address the questions that may arise.DetectoR
2. Qualitative research and quantitative analysis.
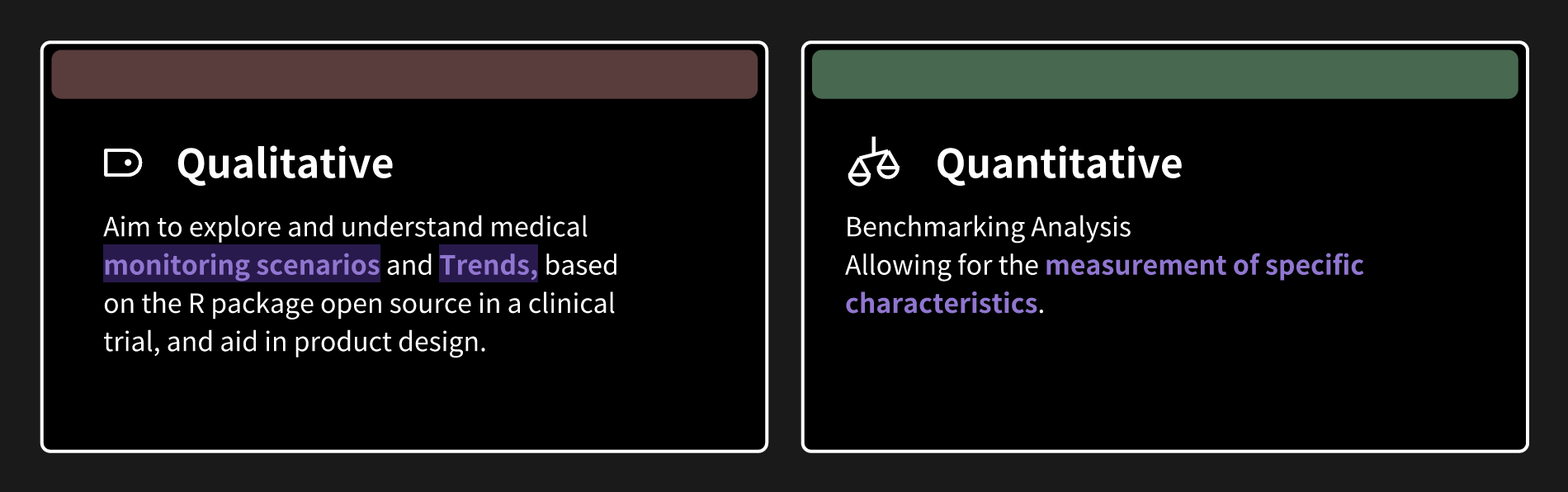


3. Propose the optimal user journey and user flow for configuring the source of Medical Monitoring and explore possible solutions.
The open-source R package can address the core concerns of DIA in medical scenarios, providing assistance in the design of product functionalities.

https://cran.r-project.org/web/packages/subscreen/index.html
Simplify sub-group analysis associated with relevant results.

https://cran.r-project.org/web/packages/subscreen/index.html
https://rdrr.io/cran/adepro/#vignettes
Animation and visualization of Adverse Event (AE) profiles
Insights into laboratory data with evolution over time
https://github.com/openpharma/elaborator
https://link.springer.com/article/10.1007/s43441-021-00318-4
https://rdrr.io/cran/elaborator/
https://pubmed.ncbi.nlm.nih.gov/34196957/
https://github.com/Bayer-Group/BIC-DetectoR
The DetectoR R Shiny app provides a handy platform allowing for early identification of signals and an
ongoing monitoring of safety along the medical product development phase and lifecycle.
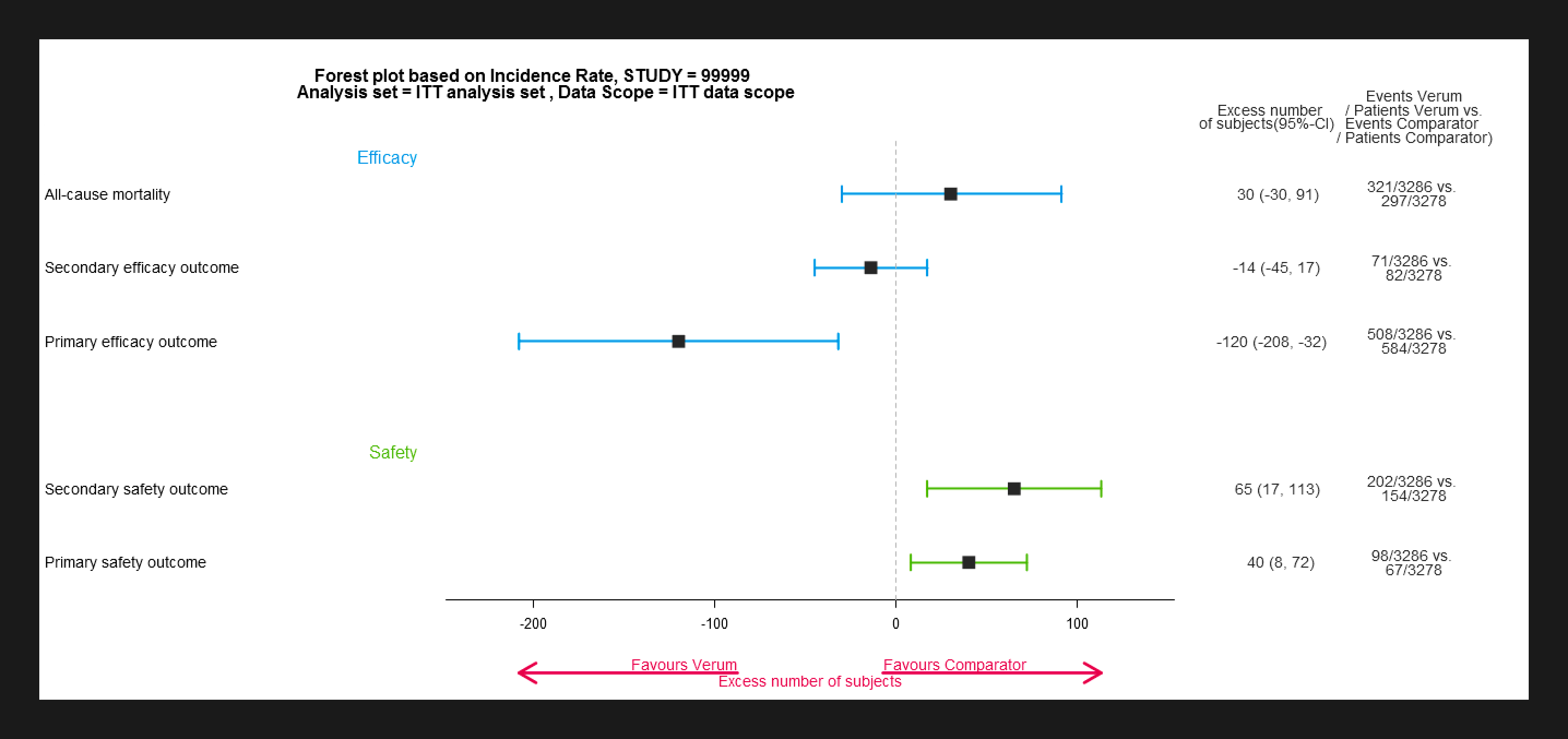
https://github.com/Bayer-Group/BIC-BReasy#breasy-
The BReasy R Shiny app provides a handy platform for structured benefit-risk assessment using clinical study
or pooled data. With the provided forest plot estimates for all relevant efficacy and safety outcomes can be
shown in one graph. In the graphical display the presented outcomes can be separated into efficacy and
safety. To enable a structured and transparent approach to assessing the benefit-risk profile, the outcomes
shown in the forest plot are based on the same analysis sets and data scopes. Furthermore, a presentation by
study (in case of pooled data), stratum and subgroups is possible to assess the benefit-risk profile in
subpopulations of interest.
Why I Use Benchmarking Analysis?
Benchmarking can provide an efficient and effective means for a firm to take the best ideas from other firms, integrate them into their own, and take actions that may provide it with a strong advantage over their competitors.
In this quantitative research, I selected five representative products in the field of medical statistics, namely SAS Viya, Plotly Dash, SPSSPRO, JMP Clinical, and Tableau. I chose them individually because they have significant influence in the life science professionals' domain and are recognized by global associations such as DIA and software user groups in life science like PharmaSUG. The factors contributing to their influence include efficiency and speed, collaboration capabilities, scalability, advanced analytics, model deployment, integration with other tools, and user-friendly interfaces.

Dimension that need to be benchmarked, and its explanation:
a. Data Management: Utilize common methods such as merging, concatenating, or joining datasets based on shared variables. Choose the appropriate join type (e.g., inner join, outer join, left join, right join) depending on the analysis nature and data relationships.
b. Data Transformation: Convert data into a suitable format and normalize or standardize it if necessary.
c. Exploratory Data Analysis (EDA): Proficiency in exploratory analysis is crucial for data scientists. They employ EDA to analyze datasets, summarize key characteristics, and utilize data visualization methods. EDA helps in determining the best approach to manipulate the data source for desired insights, facilitating the identification of patterns, anomalies, hypothesis testing, and assumption examination.
d. Interactive Graphs & Data Visualization: Data visualization is pivotal for simplifying complex datasets and effectively communicating insights. It enables users to identify patterns, trends, and anomalies, fostering a deeper understanding of the data. Ultimately, it plays a crucial role in facilitating informed decision-making by transforming data into visual representations.
e. Medical Modeling Development: Statistical models like Kaplan-Meier Survival Curve, Ridit Analysis, and Chi-Square Test are commonly used in clinical research.
f. Decision Building with AI, ML, Modeling: Clinical statisticians can leverage these tools to gain deeper insights from clinical data, identify patterns, and enhance predictive modeling in areas such as patient outcomes and treatment efficacy.
Product concept:
A powerful data visualization tool that enables users to create interactive and shareable
dashboards, allowing for intuitive exploration and understanding of complex datasets.
Pros:
1. Has basic dataset processing capabilities and strong capabilities in visualizing data.
Cons:
1. However, it performs weakly in medical scenarios and lacks accumulated experience in medical
modeling.
https://azureuse011456.my-trials.sas.com/SASDataExplorer/
Product Concept:
SAS Viya is a robust analytics platform designed to empower organizations with efficient,
collaborative, and scalable data analysis, machine learning deployment, and advanced analytics
capabilities.
Pros:
It offers advanced analytics, scalability, and efficient collaboration for diverse life science
professionals.
customization of figures.
Cons:
Its extensive functionalities may require a steeper learning curve for some users.
https://chart-studio.plotly.com/create/?fid=plotly2_demo:417#/
Product Concept:
Low/No Code mode. The interface configuration capability based on Plotly is essentially
equivalent to the coding ability in Plotly.
Pros:
1. The model structure is fixed and clear, making it highly replicable.
2. High and relatively comprehensive customization of visualization, supporting strong customer
customization of figures.
Cons:
1. Supports straightforward data processing workflows, allowing for direct analysis based on tables.
2. However, lacks the accumulation of medical industry models and insights, as well as in-depth analyses
such as curve fitting, and model prediction analysis.
https://www.spsspro.com/prodraw/operation/4632787?name=AdverseR.csv
Product Concept:
There is a complete data processing workflow. The configuration process is user-friendly,
and one can learn a lot about data flows from the platform. The approach to plotting is similar to
Tableau.
Pros:
1. The data flow processing is relatively comprehensive, providing essential data analysis workflows.
Cons:
1. Visual interactivity is not very strong.
Product Concept:
Offering advanced statistical analysis and visualization tools to streamline and enhance
the analysis of clinical trial data, facilitating data-driven decision-making in the pharmaceutical and
healthcare sectors.
Pros:
1. Emphasizing annotated visualization generation and exploratory analysis
Cons:
1. Do not emphasize data processing and manipulation.
1. The current research has not assessed the effectiveness of data results, only evaluating the product
layout from a functional and process perspective. Further in-depth research is needed to reveal the
professionalism presented by each product in the dimension of data analysis effectiveness.
2. Through research, an understanding of the complete life cycle process of data has been gained, and the
business flows generated based on this data cycle are widely recognized in the industry.

3. Mainstream, well-developed, and widely applicable data insights are based on the lifecycle of data.
Different
product lines may have slight variations in their emphasis on features.
4. The mainstream visualization configuration methods generally fall into two categories:
No Firm Does Everything the Best
1. Based on scenario templates for rapid generation, not open for user configuration, only for initialization. At a coarse granularity, to discover significant security data issues.
2. Leveraging the interface-equivalent coding capability based on the R ecosystem, providing users with a basic plot configuration end is the optimal solution while offering the capability for configuration.
1. Positioning Strategy 1: Quick launch and setup of MM plots are based on the encapsulation of business scenarios, reducing data exploration and increasing verification efficiency.
2. Positioning Strategy 2: MM Config
Proposal 1: Drag a Gallery chart here to use it as your starting point.
Proposal 2: Click on the Basic Elements tab to build a chart element by element.