Product Strategy
Construct Product Design 0 to 1 Case Retro Sharing
2021.04 - 2023.11
Researcher & Designer
20 mins
2021.04 - 2023.11
Researcher & Designer
20 mins
This article provides a brief retrospective on how the author, as an early-stage member, incubated a clinical database design tool from both top-down and bottom-up perspectives, building it from the ground up with the goal of disrupting the industry. This article reveals the deep involvement in pitching the product concept, gradually identifying the product-market fit, and progressively entering the market to acquire customers and users.
Meanwhile, using the designing logic engine tool as an example, the article provides specific insights into detailed description of the process of refining product details.
Construct is a SaaS platform that streamlines and simplifies the construction of clinical trial study design, ensuring efficient and accurate data collection and management.
Below, I've provided an end-to-end (E2E) demonstration to assist you in understanding how Construct operates.
As of today, in just a short span of 2 years since its establishment, the Construct product line has successfully garnered numerous major clients (Big Logo) in the Chinese market, including clients such as RENGEN Biotech, Tiger Med, HXYT, and more. And moreover, it is progressively advancing into the global market.
Because of its excellent usability and user experience, which spinning the wheel Of Product Growth By Design.
Proven Customer Success story.
The process primarily employed the "as-is" and "to-be" methods. It involved documenting and analyzing the current state or processes within a system or organization, providing a baseline understanding of the existing situation. On the other hand, it outlined the desired future state or improvements, offering a vision of the intended changes and guiding the transition from the current state to the envisioned state.
What trends are influencing our addressable market and what’s the size and shape of the resulting commercial opportunity?
A growing number of pharmaceutical and biotechnology companies and contract research organizations have taken steps to optimize their protocol designs in order to improve feasibility, ease site and subject participation burden, reduce the number of unplanned and unbudgeted protocol amendments, and gather more meaningfuI clinical data. These initiatives include protocol review committees, protocol-authoring practices connecting procedures to primary and key secondary end points, common protocol-authoring templates, and soliciting feedback on draft protocol designs frorm patients and investigative site staff before approval and execution.
How might the underlying technology evolve to support the needs of our customers and enable our commercial strategy?
Entering this industry, we realize that it is facing increasingly complex trial designs. The industry trend is moving towards simplifying Clinical Study Design through Computer-Aided Design (CAD). This shift is actively transforming the clinical industry towards digitization while complying with regulations. This appears to offer entry points for startup companies new to the industry.
Additionally, from a user perspective, we often observe that those involved in the design and implementation of clinical trials frequently use flowcharts to explain the logical sequence and processes of the trials to relevant personnel.

Our users require an interface on the terminal that supports them in achieving faster and more standardized experiment design.
Therefore, the first Proof of Concept (POC) was born: Study Flow Builder. The concept is to link trial design with traditional product matrices and form design by selecting the trial methods, making the trial design clearer and more intuitive. For example, embedding the configuration of classical randomization schemes into the configuration process simplifies these complex experimental configurations, making them more straightforward, standardized, and less reliant on personal experience. This greatly streamlines the trial design process, reducing the threshold for trial design.
Demo: Proof of Concept for the Study Design Project. Showcased at the inaugural product launch event, X-day Break, to convey the value and determination we aim to bring to the industry.
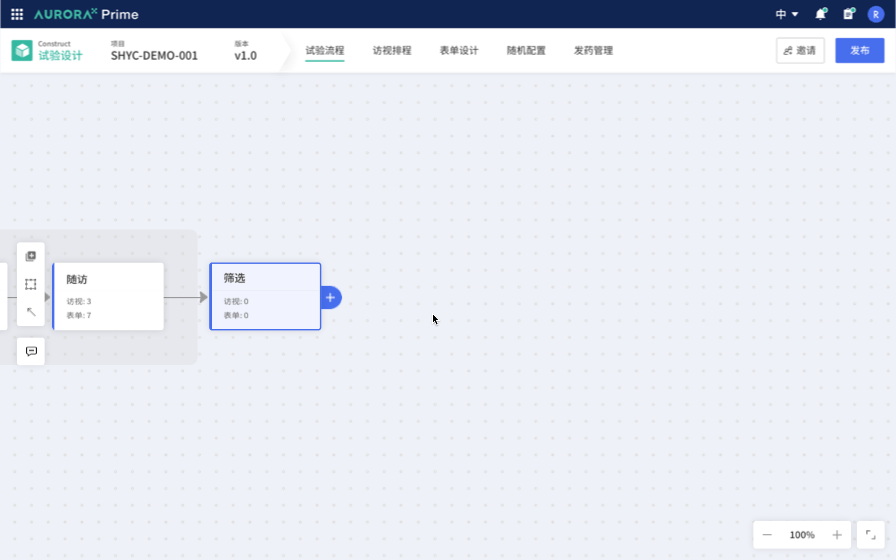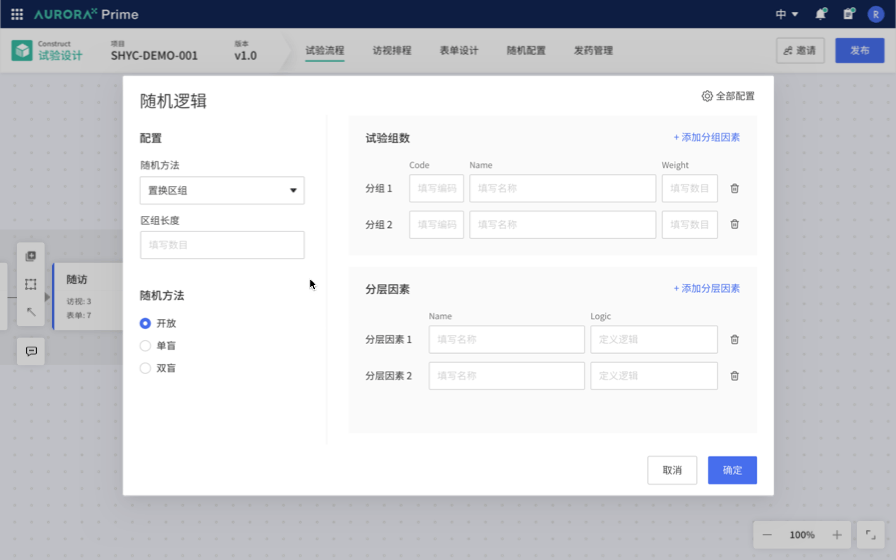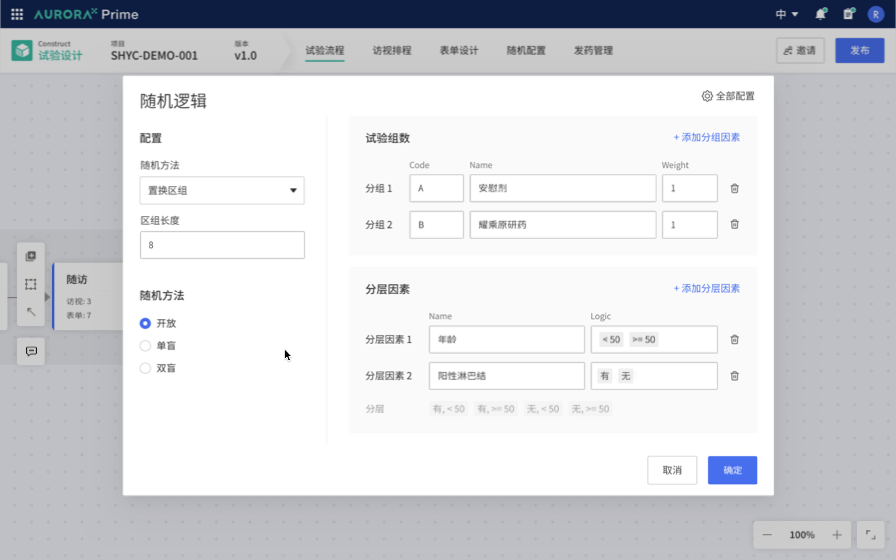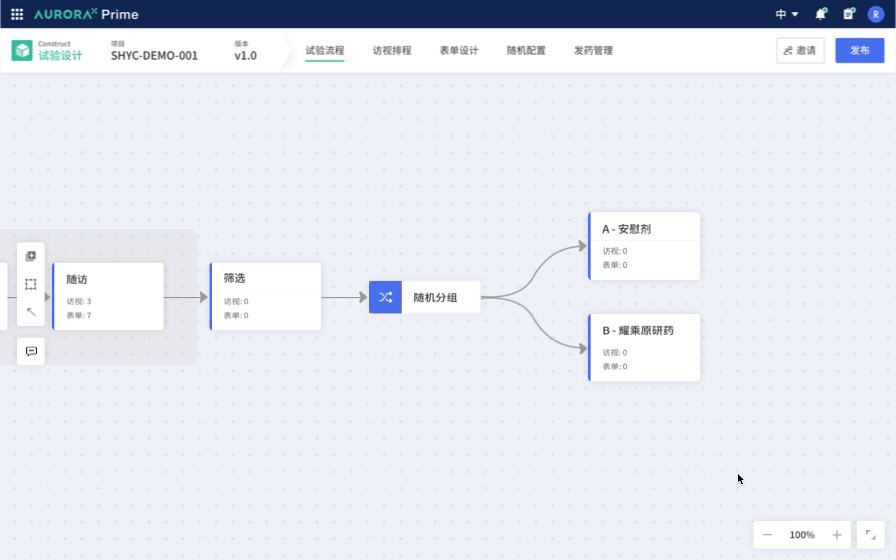
This vision is very promising. We recognize industry trends, identify unresolved needs, and believe that, in the field of Computer Science, we excel at addressing such issues. While being a technology-driven startup, we currently lack the industry-specific knowledge and experience to quickly identify the best practice solutions for industry pain points.
In the early stages of going from 0 to 1, we are re-immersing ourselves to continue finding the Product-Market Fit and establish a solid foundation for the product. Through multiple rounds of pitching with clients, we have adjusted our criteria for opportunities and alignment with clients: focusing on what startups excel at - driving industries with technology, meeting the immediate needs of existing clients, and addressing some of the unresolved needs with innovative solutions.
Therefore, we propose: Identify unmet product needs for the lower-end market, utilize pricing advantages to gain development space, and enhance product performance and services. Subsequently, migrate towards higher-end market customers.
In the clinical industry, EDC suites have become the industry-standard suites. Therefore, we plan to replicate standardized suites and explore points of local innovation within the standard processes to enhance our product workflows.
In the existing process, a challenging aspect was the writing of logic. DBD aimed to ensure that data complied with trial logic by rapidly creating Edit Checks. However, for complex logic such as time window comparisons (checking if the time is within a 5-minute absolute value of the administration time, triggering an EDC query otherwise), Custom Function Scripts needed to be written. This increased the entry barrier for industry professionals and slowed down the trial implementation process.
Opportunity: We aim to provide standardization of processes for professionals in the clinical industry, particularly those who in Data Management (DM) and Database Designer (DBD). By employing multi-dimensional tool selection, we aim to lower the entry barriers for practitioners, starting from the lower end of the market, and address unresolved needs. The primary objective is to significantly reduce the cost of development cycle in clinical trials, creating more value for Clinical Contract Research Organizations (CROs) and pharmaceutical companies while securing profit margins in the clinical industry.
Therefore, we aim to allow Database Designers to write such extensively reusable logic through a visual interface, opening up a Low code / No Code interface. We envision using declarative language to replace scripts, enabling practitioners to focus on understanding the business without being bound by code and facilitating trial deployment. Consequently, we have outlined the details of the Edit Check local innovation proof of concept.
Design Concept_01: Functionality Design involves constructing logic statements by assembling modular components on the left side. Different colored blocks are used to differentiate functional modules, achieving an intuitive construction of logic.
Oberservation: While solving the pain point of making the writing process more intuitive, the drawbacks of the solution became apparent. The drag & drop approach, while composing logic becomes more intuitive, but did not improve efficiency. This prompted design solution iterations.
Design Concept_02: Keyboard-only user experience, typing by composing. I always strive to deliver intuitive and user-centered solutions to our end users.
Take a glance at the design I created below. It is interactive, catching users' attention all the way all the time by providing timely hint and support(Perceivable Affordance) for our end users to compose a valid expression. This solution is infused with fascination throughout the journey. Make boring daily work fun! Fostering a sense of accomplishment in their work.

In order to ensure that the designed tool aligns with intuition, achieves the goal of enabling users to quickly get started, and significantly improves efficiency, we conducted various validation methods and activities:
👉🏻 Check it: Event Review | X-Innovator Community Workshop 2022Q1
1. Remote and In-person interview: Conducting both remote and in-person interviews. Gather initial insights from a diverse user base or a subset of users to understand their needs, pain points, and delve deeper into their experiences, preferences, and motivations when conductiong in-person interviews.
2. Multiple rounds of user validation were conducted, involving various CRO DBDs and Data Managers. Logic Engine tasks were assigned to calculate task completion time and success rates. User feedback was consistently recorded during this process.
3. A two-day workshop, X-Innovator Community Workshop 2022Q1, was organized. Beginning with user training followed by a logic-writing challenge. The challenge simulated real database construction scenarios, providing participants with a Cheatsheet for reference. User feedback was documented throughout the process.
👉🏻 Check it: Event Review | X-Innovator Community Workshop 2022Q1
After multiple rounds of validation and iterations, Logic Composer was successfully launched. It not only aligns with user intuition but also reduces the amount of code needed for logic writing, lowering the entry barrier. Simultaneously, the efficiency of writing individual logics has significantly improved. The Construct product line reached its first milestone with the introduction of self-built database users utilizing the product and tool, contributing to the company's revenue.
Compare the CRF (Case Report Form) creation process between the Construct product and the Medidata Rave product pages. It can be observed that, despite having a significant market share, the user experience flow for trial design in the established product involves multiple rounds of page navigation. This could be considered a rather poor user experience.
Construct Fast go-live strategy effects: Efficiency Improved By 70%.
How might the product/product experience evolve to support the needs of our target customers and enable our commercial strategy?
knowing the people we’re trying to reach, what they need and how they behave and mapping that back to the current offering and resulting experience. This analysis provides the backbone of our strategy and is used to rationalise and drive a series of longitudinal design decisions; each paving the way towards a better, commercially aligned future.

1. I consistently rely on my keen, discerning, and savvy product intuition, coupled with creativity and a penchant for tinkering, to explore the potential forms of a product and determine what works.
2. I have efficiently planned and implemented the utilization of design strategy, leveraging strategic thinking to facilitate the continuous optimization and iteration of design approaches. This has led to the continual enhancement of the design team's capabilities and the establishment of long-lasting collaborations with clients.
3. I have conducted comprehensive research, consistently assisting the team in understanding the target audience and stakeholders for the services we offer.
4. I have excelled in communication, adeptly balancing conflicting interests among all stakeholders, enabling the team to consistently propose innovative solutions to challenges.
5. I have demonstrated excellent time management, ensuring the smooth implementation of phase objectives consistently.
1. I don't have enough compelling reasons yet to convince the tech team. I am considering exploring more cost-effective technologies to achieve product validation, aiming to further shorten the validation cycle.